The Obesity and Cancer Link – Unraveling molecular interconnections and identifying potential therapeutic targets of significance
The link between Obesity and Cancer has emerged as a significant risk factor for a range of malignancies, including melanoma, endometrial, prostate, pancreatic, esophageal adenocarcinoma, colorectal carcinoma, renal adenocarcinoma, and pre-and post- menopausal breast cancer.
A narrative review published by researchers from Qatar, Saudi Arabia, UAE, and India offers a comprehensive examination of the molecular interconnections between obesity and cancer and potential therapeutic strategies by integrating and synthesize findings from various studies.
Obesity’s association with cancer development
Researchers estimate that obesity plays a role in approximately 20 % of all cancers. Epidemiological evidence strongly supports the link across numerous cancer sites, most notably:
- Breast Cancer: Obesity increases the risk of developing postmenopausal breast cancer by approximately 30%. This risk is particularly high for estrogen receptor-positive (ER-positive) breast cancer in postmenopausal women.
- Colorectal Cancer (CRC): Persistent obesity is a strong, independent risk factor for CRC in both sexes, increasing the risk by 18% to 32%. Gut microbial imbalance (dysbiosis), which can be induced by an obese individual’s microbiota plays a major role.
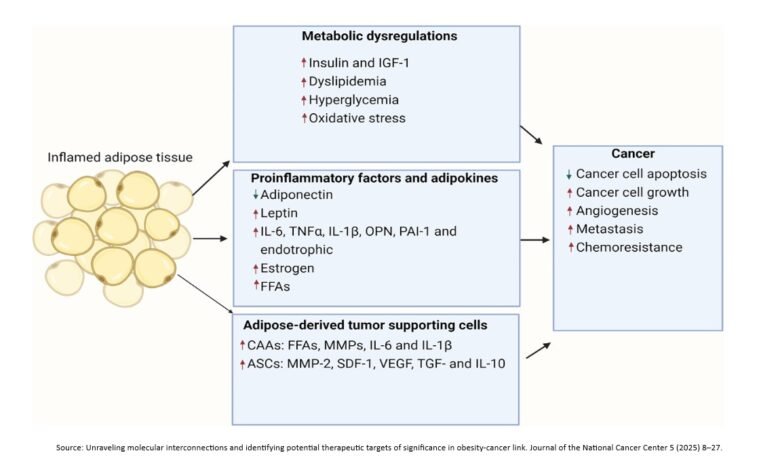
Molecular interconnections between obesity and cancer
Metabolic perturbations, chronic inflammation, and growth factor imbalances are the three primary contributing patho-physiological elements responsible for the increased cancer risk observed in obesity. These contributors also significantly worsen cancer prognosis and treatment response.
- Obesity causes metabolic disruptions like pancreatic steatosis and non-alcoholic fatty liver disease, increasing inflammation and cancer risk. Certain FTO (Fat Mass and Obesity-associated) gene variants are consistently linked to increased risks of various cancers.
- Metabolic reprogramming in obese individuals, marked by hyperglycemia, dyslipidemia, and insulin resistance, accelerates cancer cell proliferation. Hyperinsulinemia activates the Insulin Growth Factor (IGF) pathway, and elevated IGF-1 is linked to increased cancer risk.
- Obesity also disrupts lipid metabolism, and dysregulated transport and synthesis of lipids are hallmarks of cancer. Furthermore, elevated levels of adipokines like leptin, resistin, and visfatin are crucial mediators of obesity-driven carcinogenesis, promoting inflammation, angiogenesis, and cell growth.
- Cellular metabolism is tightly regulated. Increased obesity creates an imbalance resulting in heightened oxidative stress (ROS), fueling tumor progression.
- In the Tumor Microenvironment (TME), obesity transforms adipocytes into a cancer-associated phenotype, releasing pro-tumor cytokines (IL-6, IL-1β) and free fatty acids that fuel tumor growth.
- Extracellular vesicles from obese adipose tissue also mediate pro-tumoral signaling, enhancing cancer migration, invasion, and resistance.
Obesity’s Impact on Cancer Treatment
Obesity significantly worsens cancer prognosis and hinders the effectiveness of most cancer treatments (chemotherapy, targeted therapies, radiation). This is due to:
- Pharmacokinetic changes (altered drug distribution and metabolism).
- A chronic inflammatory microenvironment (elevated pro-inflammatory cytokines).
- Direct adipocyte-cancer cell interactions that promote tumor survival.
However, the “obesity paradox” is a notable exception where some obese patients show improved responses to immune checkpoint inhibitors, possibly because chronic inflammation creates an immune-stimulatory environment.
Therapeutic interventions targeting the obesity-cancer connection
- Metformin (Glucophage), a widely used antidiabetic drug, shows promise in reducing the incidence and mortality of several cancers. It improves insulin sensitivity, lowers circulating insulin, and inhibits oncogenic pathways.
- Adiponectin, a hormone, exhibits anti-proliferative and anti-angiogenic properties. Higher levels are inversely correlated with several cancer risks, as it deactivates tumor growth pathways and reduces chronic inflammation, mitigating the obesity-cancer link.
- Emerging strategies include Targeting Epigenetic Modifications by inhibiting FTO.
- Anti-inflammatory Interventions including lifestyle changes, pharmacological agents, and natural compounds are crucial, as chronic inflammation drives both obesity and cancer progression.
The connection between obesity and cancer is complex, involving insulin resistance, adipokines, and chronic inflammation. Addressing adipose tissue inflammation and developing personalized treatments are crucial for improving outcomes for this patient population. Future research should aim to move beyond Body Mass Index (BMI) as a sole measure, integrating a wider range of metabolic health biomarkers to better define and address obesity-associated cancers, ultimately paving the way for personalized cancer management strategies.
Reference Source
Alanoud Abdulla, Hana Q. Sadida, Jayakumar Jerobin, et al. Unraveling molecular interconnections and identifying potential therapeutic targets of significance in obesity-cancer link. Journal of the National Cancer Center 5 (2025) 8–27. https://doi.org/10.1016/j.jncc.2024.11.001







